Funding for C3FIT Trial Approved by PCORI
Recently, the board of PCORI approved funding for the Comparative Effectiveness Randomized Trial to Improve Stroke Care Delivery: C3FIT: Coordinated, Collaborative, Comprehensive, Family-Based, Integrated, and Technology-Enabled Care. The PCORI summary is here. I’m pleased to be serving on the stakeholder engagement committee for the study.
Talk at CVCT 2017
I was invited to give a brief talk as a patient advocate at Cardiovascular Clinical Trialists Forum. Here are my slides.
Salim Yusuf on nutrition and cardiovascular disease
Addendum Feb. 21, 2017. It has come to my attention that the video of the talk by Salim Yusuf I linked to above has been taken down. I don’t know why the video was taken down, but I will update this post if/when I find out.
Addendum Feb. 27, 2017. Larry Husten has written a summary of the talk.
Addendum March 4, 2017. Some experts quoted criticizing some of Yusuf’s points.
LDL-Lowering Genetic Variants Linked to Increased Risk of Diabetes
–What can genetic studies tell us about the risk of developing diabetes with cholesterol-lowering therapies?
This post was first published on MedPageToday and CardioBrief.org.
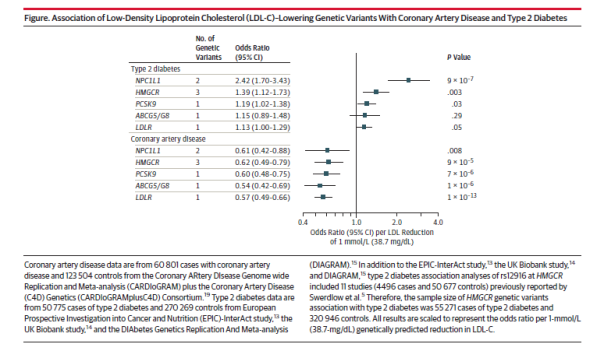
In a study published in the Journal of the American Medical Association, several LDL-lowering genetic variants were found to be associated with a reduced risk of coronary artery disease and an increased risk of type 2 diabetes. The study investigated LDL-lowering alleles in or near Niemann-Pick C1-Like 1 (NPC1L1), HMG-CoA reductase (HMGCR), PCSK9, ABCG5/G8, and low density lipoprotein receptor (LDLR). NPC1L1 is the target of ezetimibe, while HMGCR is the target of statins and PCSK9 is the target of PCSK9 inhibitors.
Luca Lotta, of the University of Cambridge, and colleagues conducted meta-analyses of genetic association studies, and included 50,775 individuals with type 2 diabetes and 270,269 controls and 60,801 individuals with CAD and 123,504 controls.
The study found that for a 38.7 mg/dL reduction in LDL-cholesterol, the genetic variants were associated with a similar reduction in risk of coronary artery disease, with odds ratios ranging from 0.54 to 0.62. However, genetic variants at the NPC1L1 locus were associated with a higher risk of diabetes (odds ratio 2.42) as compared to controls than the HMGCR and PCSK9 genetic variants (odd ratios of 1.39 and 1.19, respectively). The type 2 diabetes findings for NPC1L1 and HMGCR were highly significant (p = 9 x 107 and p = .003, respectively), but the p value for the type 2 diabetes finding for the PCSK9 variants was .03. The associations with type 2 diabetes for ABCG5/G8 and LDLR were not significant.
Treatment with statins is known to be associated with a higher incidence of new-onset diabetes, as is treatment with niacin, but the effect of ezetimibe and PCSK9 inhibitors on new-onset diabetes is unclear. An analysis of the IMPROVE-IT trial showed a small increase in new-onset diabetes in the ezetimibe group, but the difference was not statistically significant. The published data for the PCSK9 inhibitors have not shown statistically significant increases in blood sugar or new-onset diabetes (see here and here), but much more data will be available when the PCSK9 inhibitor outcomes trials are completed, starting next year.
One reason to wonder why PCSK9 inhibitors might increase blood sugar is that both statins and PCSK9 inhibitors have mechanisms of action that involve the removal of LDL from the bloodstream through upregulation of the LDL receptors. A recent study showed that patients with familial hypercholesterolemia, a disease that involves dysfunction of the LDL receptors, have a lower prevalence of type 2 diabetes as compared to their unaffected relatives. The study suggests that LDL receptor function may be involved in glucose homeostasis. I asked several experts in clinical trials or cardiovascular genetics to comment by email.
Sanjay Kaul, MD, of Cedars Sinai Medical Center in Los Angeles, sent the following comment by email:
The association of LDL-lowering alleles with CV risk is consistent across the 5 alleles. However, the association of LDL-lowering alleles with risk of T2DM is confined to NPC1L1 (large effect size, HR 2.4 and statistically robust p value) and to a lesser extent to HMGCR (HR 1.4, p = 0.003). This finding suggests an increased risk of incident diabetes with ezetimibe that targets NPC1L1. However, the incidence of new onset DM (defined as initiation of anti-diabetic medication during trial or two consecutive fasting glucose ≥126 mg/dL) in IMPROVE-IT trial was 720/5297 (13.6%) in EZ/SV vs. 694/5341 (13.0%) in SV, HR 1.04 95% CI (0.94, 1.15). The additional LDL lowering with ezetimibe was approximately 16 mg/dL which translates into a HR of 1.10 per mmol/L LDL lowering (1.04/16) x 38.7). This is considerably lower than the HR of 2.4 observed in the gene association study. Of course the median exposure in IMPROVE-IT was only 7 years when about 42% of subjects had discontinued treatment compared with the lifetime exposure in the gene association study. One would need a larger data set (meta-analysis of SHARP, SEAS, IMPROVE-IT, ARBITER-6, etc.) to better characterize the risk of incident T2DM. Even if we assume the association to be causal, remember the treatment effect in IMPROVE-IT was exclusively confined to the diabetic cohort who comprised 27% of the overall cohort (HR 0.86, 95% CI 0.78, 0.84 vs HR 0.98 for the non-diabetic cohort).
Joshua Knowles, MD, PhD, of Stanford University in California, sent the following comment:
This is an important paper by a very good group of investigators. The overall results of this Mendelian randomization study are not that surprising but are still very important, that there is an inverse relationship between LDL-C lowering genetic alleles and risk of type 2 diabetes.
There has been a lot of evidence emerging about this from the large statin trials to studies of [familial hypercholesterolemia (FH)] patients to prior Mendelian randomization studies.
The fact that they observe some heterogeneity of effect is interesting in that it might suggest that different ways of lowering LDL-C might result in different levels of risk for type 2 diabetes.
The overall effect they see for NPC1L1 genetic variants (a risk of 2.42 for type 2 diabetes for every 1 mmol/L reduction in LDL-C) suggests that this mechanism might theoretically be more potent for causing T2D risk.
However, in practice, ezetimibe does not lower LDL-C by 1 mmol/l but more like by ~0.5 mmol/L (or even less) so the actual effect size in ezetimibe trials (like IMPROVE-IT) will be less than 2.4 (probably more like 1.2). And in IMPROVE IT the effects might be masked to some extent as everyone was also on a statin and we don’t know if the effects would be additive.
The large scale PCKS9 trials will be revealing for their risk. Certainly these studies do not suggest that there will be a big effect which is good for the patients taking them now.
Please emphasize that overall message remains that for high risk patients (like FH) the beneficial effect of LDL-C lowering will greatly trump the increased risk of type 2 diabetes.
What is fascinating for me is we really have no idea whether the increased T2D risk is because the drugs decrease insulin secretion or increase insulin resistance. Knowing this will be critically important.
I am very interested in this topic and have a Doris Duke Clinical Investigator Grant to study it in a randomized trial. We will be measuring (with gold standard measures) insulin secretion and insulin resistance pre and post statin.
Remember that T2D is simply defined as an increase in blood glucose. These drugs seem to mostly push people that are ALMOST diabetic just over the threshold (see our recent paper published in the American Journal of Cardiology). Simply having an average blood glucose level go from 123 mg/dl to 127 mg/dl probably is not that important to a single person’s individual risk of downstream bad outcomes (though that person would go from being a pre-diabetic to a diabetic with that small change in blood glucose). What may be more important is HOW and WHY that blood glucose level rose. If there is not enough insulin being made, the treatment would potentially be different than if the body is not responding to insulin.
Another key message is to reemphasize the importance of exercise and maintenance of a healthy weight to potentially counteract the effect of these LDL-C lowering drugs on T2D. We should continue to advocate those important lifestyle choices for our patients. If you look at the data in [our paper] the risk of T2D with a statin is EXTREMELY low (3%) in those with normal fasting glucose and normal triglycerides (or weight) but very high in patients with pre-diabetes and high triglycerides (or overweight)– 23%!
I also received this comment from Daniel Swerdlow, MBBS, PhD, of Imperial College London:
This is a well-designed analysis that uses methods that are now established for using genetics to explore the effects of drug target modulation. The associations of the variants in NPC1L1 and PCSK9 on type 2 diabetes risk are not unexpected, as it appears from other large genetic analyses published recently that LDL-C lowering associates with higher diabetes risk, regardless of the mechanism through which this is achieved. This has been borne out in trials of statins and niacin, though the IMPROVE-IT trial of ezetimibe did not demonstrate an increased risk of diabetes in the treatment arm. The effect sizes in studies such as this are less informative than the direction of the effect, since direct comparison of the magnitudes of genetic effects and drug treatments is restricted by the differences in duration and potency of the two ‘interventions’. The biological analogy, however, allows the directions and scope of genetic associations to be interpreted as proxies for drug effects on the target encoded by the gene in question. The issue of new onset diabetes risk is pertinent for the PCSK9 inhibitors, and although analyses of trial data to-date has shown no association, they have been limited by duration of follow-up and sample size. The large phase 3 outcome trials are expected to focus carefully on diabetes risk with these new agents.
The over-riding message that analyses such as this in JAMA emphasize is that lipid-modifying treatments are only one part of cardiovascular risk reduction, and must be accompanied by lifestyle modification, such as appropriate diet and higher physical activity, in order to optimize risk reduction and mitigate against the small increase in diabetes risk that has been shown to be associated with some lipid-modifying drug treatments.
Diminishing returns in medical therapy
I’m a few months late, but I want to mention an editorial by Rodney Hayward that was published in The BMJ in December 2015. His topic is treatment of diabetes, but the principles he discusses also apply to other areas of medicine. The key concept is that even in high risk conditions such as diabetes, adding a second or third medication brings diminishing absolute returns as residual risk decreases as each additional treatment is added. He starts by describing the disturbing consequences of untreated or poorly managed diabetes, and how things have changed with modern therapies.
When I began my medical training in 1980, I commonly encountered patients whose bodies were ravaged by end stage complications of diabetes. These patients often had marked visual impairment, debilitating neuropathy, myopathies, and diabetes related renal insufficiency, well before age 65 years. I still occasionally see such individuals, but they are rare, and tend to come from the 10-15% of patients who still have poor glycemic control. Improvement in diabetes care is a medical success story, but increasing evidence suggests that overly aggressive treatment is an under-appreciated problem.
The problem is that focusing on relative treatment effects ignores the law of diminishing returns; past a certain point, additional reductions in HbA1c have limited benefit in absolute terms for most older patients with type 2 diabetes. Hayward explains:
Diminishing returns is a mathematical fact, not a theory. Try this simple experiment. Serially tear a piece of paper in half and throw one half away. You will notice that the relative effects never diminish (you reduce the piece of paper by half each time), but it doesn’t take long for the 50% you throw away to become tiny. The many patients with end stage diabetes we saw in the 1980s often spent years with poor control of both glycemia and blood pressure. They had no access to metformin, home blood glucose monitoring, angiotensin converting enzyme inhibitors, calcium channel blockers, and a host of other modern interventions. Each of these interventions substantially reduces disease progression and has an even larger effect on end stage diabetes complications. Because each intervention substantially reduces end stage complications, it should not be surprising that recent evidence has found intensive glycemic control to have a small absolute effect on end stage complications for most patients with type 2 diabetes. The law of diminishing returns predicts this result. Also, as the benefits of tighter glycemic control become smaller, the chances that treatment harms will outweigh treatment benefits become much greater.
Hayward ends by stating that the public good would best be served by focusing on the minority of diabetes patients who continue to be at substantial risk of diabetes-related morbidity and mortality and promoting more shared decision making with older diabetes patients who already have at least moderate blood glucose control.
The same principle of diminishing returns applies in other areas of medicine, such as in medications that reduce cardiovascular risk by lowering blood pressure or cholesterol. As the second and third medication is added, the patient’s risk of experiencing a cardiovascular event diminishes and in some cases a point can be reached where the absolute benefits become very small and it becomes difficult to tell whether benefits outweigh harms. When benefits become small, it can sometimes be hard to determine whether they exist at all or, perhaps, exist in only in patients with certain characteristics. See this post by Harlan Krumholz for a discussion of these issues in the area of treatment of high blood pressure.
In the area of cholesterol-lowering drugs, the new PCSK9 inhibitors have been in the news and I’ve previously discussed them on this blog (here, here and here). Two of these drugs, evolocumab and alirocumab, are approved in the U.S. and so far aren’t selling well. There are several reasons for that, including that the outcomes trials haven’t been completed yet and that the drugs are much more expensive than statins, almost all of which are available as generics. Another reason, related to the first two, is that insurance companies have imposed strict preauthorization requirements for these drugs. Another reason relates to the theme of this post, namely the diminishing returns from adding additional drugs. I’m going to take the treatment of heterozygous familial hypercholesterolemia (HeFH) as an example, specifically patients with HeFH who do not have clinical atherosclerotic cardiovascular disease and who are thus being treated to prevent a first event (i.e., “primary prevention”).
HeFH greatly increases the risk of developing premature atherosclerotic cardiovascular disease compared to individuals with normal levels of cholesterol. Before statins became available, the drugs that were available were not very effective. However, in recent decades, first moderate intensity and then high intensity statins were instituted as standard treatment of HeFH, often with additional drugs such as ezetimibe. According to UpToDate, atorvastatin can reduce LDL by up to 54% and rosuvastatin can reduce LDL by up to 63%. Ezetimibe can lower LDL by another 15% or so in patients on a statin. Given that most patients with HeFH have LDL in the 200s or below, a reduction of 50-60% achieves very reasonable LDL levels. There is evidence that even moderate doses of statins greatly reduce the risk of heart disease in HeFH patients who are being treated for primary prevention. A study published in JAMA in 2014 showed that young adults with HeFH have near-normal levels of atherosclerosis 10 years after initiation of statin therapy. The use of high intensity statins has been shown to greatly reduce the progression of atherosclerosis in adult HeFH patients (see here and here) even when compared to moderate statin therapy. Thus, HeFH patients who start treatment early and are able to reduce their LDL to normal or near-normal levels over many years with a statin or statin + ezetimibe often do not need an additional drug, as their risk is greatly reduced.
So which HeFH patients do need an additional LDL-lowering therapy, such as a PCSK9 inhibitor? To my knowledge, there are no risk calculators available to guide decisions in this area. HeFH patients who start with very high LDL, who can’t tolerate high doses of statins or can’t tolerate statins at all, who started treatment late, who have additional cardiovascular risk factors, or who have had imaging that shows significant subclinical atherosclerosis, are going to be at higher risk, on average. There is quite a bit of uncertainty involved, as with estimation of cardiovascular risk in general. In addition, there are personal preferences involved, as people vary greatly in terms of how much risk they are willing to live with.
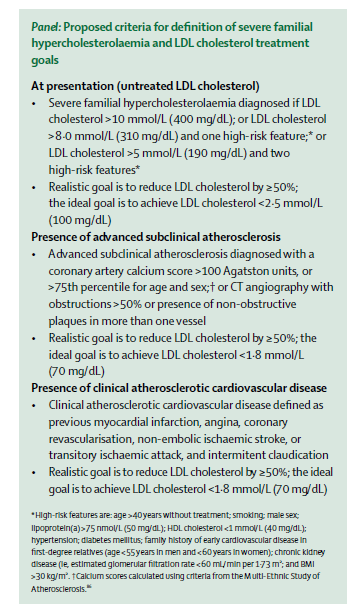
Interestingly, a task force of the International Atherosclerosis Society just published a consensus statement in The Lancet Diabetes & Endocrinology that discusses some of the factors involved in determining cardiovascular risk in FH. Although the criteria they propose for use of additional therapies are more stringent than I foresee being adopted in the U.S., the paper contains some very useful discussion of the heterogeneity of cardiovascular risk in FH and ways of trying to predict who is at higher risk. I’m pasting in their proposed criteria below, in case anyone is interested, but I do recommend the entire paper.
More data on alirocumab and diabetes risk
I happened to see an abstract from the American Heart Association Scientific Sessions in which the authors slice and dice the alirocumab data to see if they could find effects on development of diabetes or impaired glucose control in patients without diabetes at baseline. As you can see in the table below, there are some numerical differences in (1) patients with normal blood sugar at baseline who developed impaired glucose control and (2) patients with impaired glucose control who developed diabetes. The differences were not statistically significant. No resolution of this issue is likely until the results of the outcomes trials are available. See my previous post on this here.

2013 Gina Kolata article on PKSK9 inhibitors was inaccurate
A 2013 front page New York Times article by Gina Kolata on PCSK9 inhibitors was inaccurate. Here is Kolata’ lede:
She was a 32-year-old aerobics instructor from a Dallas suburb — healthy, college educated, with two young children. Nothing out of the ordinary, except one thing.
Her cholesterol was astoundingly low. Her low-density lipoprotein, or LDL, the form that promotes heart disease, was 14, a level unheard-of in healthy adults, whose normal level is over 100.
The reason was a rare gene mutation she had inherited from both her mother and her father. Only one other person, a young, healthy Zimbabwean woman whose LDL cholesterol was 15, has ever been found with the same double dose of the mutation.
Here is an excerpt from the FDA clinical review of Sanofi/Regeneron’s PCSK9 inhibitor alirocumab:
We are aware of three cases of individuals homozygous (or compound heterozygous) for loss-of-function PCSK9 alleles with very low LDL-C concentrations that have been reported in the literature:
1. a 21-year-old African woman with an LDL-C of 15 mg/dL; no further information about this patient was provided, except that she was identified for genotyping at a postnatal clinic,
2. a 32-year-old African American woman with an LDL-C of 14 mg/dL; she is an apparently healthy, normotensive, fertile, college-educated individual with normal liver and renal function tests, and
3. a 49-year-old French white man who was found to have extremely low LDL-C (7 mg/dL) on admission for rapid-onset of an insulin-requiring diabetes mellitus of unknown etiology; LDL-C not during acute illness was reported to be 16 mg/dL. This patient was shown to have moderate liver steatosis on abdominal ultrasound with normal hepatic enzymes and liver function tests. He had no reported history of diarrhea, eye, or neurological abnormalities related to any vitamin deficiency. His mother was deceased at age 66 from dementia, whereas his father was healthy at age 79. His grandparents died at the ages of 79, 87, 91, and 94 years.
At this time there are too few cases to provide conclusive data about loss-of-function PCSK9 polymorphisms and the risk of human disease, although given the association of statins with diabetes risk, the development of diabetes in the 49-year-old man discussed above is of interest. (See Dr. Roberts’ safety review for further discussion of alirocumab and glycemic parameters).
The third case is the one missed by Kolata. It was published in Arteriosclerosis, Thrombosis, and Vascular Biology, an American Heart Association journal, in 2009, and could have been found with a PubMed search. The case is interesting in that it conflicts with one of the oft-repeated but inaccurate narratives with respect to PCSK9 inhibitors, the idea that all known persons with extremely low LDL due to having two PCSK9 loss-of-function mutations are completely healthy. I agree with the FDA reviewer that there are too few such cases to provide conclusive data about loss-of-function PCSK9 polymorphisms that result in extremely low LDL levels and the risk of disease. (The issue of whether alirocumab increases blood glucose and the risk of developing diabetes is also discussed extensively in the review, with the reviewer concluding that the evidence is inconclusive at this point.)
Do PCSK9 inhibitors affect diabetes risk?
Two PCSK9 inhibitors, evolocumab and alirocumab, are under consideration at the FDA and will be the subject of advisory committee meetings on June 9 and 10. Evolocumab and alirocumab are monoclonal antibodies that inhibit proprotein convertase subtilisin/kexin type 9 (PCSK9), an enzyme that plays a role in regulating levels of LDL cholesterol by binding to LDL receptors and promoting their degradation; the resulting reduction in LDL receptors reduces the liver’s ability to remove circulating LDL. PCSK9 inhibitors prevent PCSK9 from degrading LDL receptors; the increased LDL receptor density results in increased clearance of LDL from the bloodstream. The expectation is that lower LDL levels in patients who receive PCSK9 inhibitors will result in a reduction in cardiovascular events and this strategy is currently being tested in large outcomes trials, which will be completed in a few years. Until those trials are completed, the safety and efficacy of these drugs will not be known.
One of the unknowns with PCSK9 inhibitors is their effect (if any) on blood glucose levels and the development of new-onset diabetes. Statins are known to increase the risk of new-onset diabetes by about 9% overall, with increased risk from intensive vs. moderate intensity statin therapy. One reason to wonder whether PCSK9 inhibitors might have a similar effect is that both statins and PCSK9 inhibitors, though having different mechanisms of action, both involve the removal of LDL through upregulation of LDL receptors. The reason statins increase blood glucose is unknown, but recently it has been suggested that that the LDL receptor might be involved, with greater LDL receptor activity correlating with a higher risk of diabetes. A recent study showed that patients with familial hypercholesterolemia (FH) in the Dutch FH registry have a lower prevalence of type 2 diabetes as compared to their unaffected relatives. FH is a genetic disease in which the LDL receptor function is reduced, leading to higher serum levels of LDL cholesterol. In addition, the study found a dose-response relationship, with more severe FH mutations linked to lower risk of diabetes as compared to less severe mutations. In other words, the study showed an association between less functional LDL receptors and a lower prevalence of type 2 diabetes. In an editorial, David Preiss and Naveed Sattar note that the study suggests that “the expression and function of LDL receptors may be important for glucose homeostasis” and that the advent of PCSK9 inhibitors provides an opportunity to further examine a possible link between LDL receptor expression and glycemia and diabetes risk.
I’ve looked at some of the published data on PCSK9 inhibitors and blood glucose and diabetes risk. With respect to alirocumab, I abstracted a subset of the data in an abstract presented at the March 2015 American College of Cardiology conference.
As you can see, the data show small numerical increases in new-onset diabetes and worsening of preexisting diabetes, as well as larger increases in fasting glucose and hemoglobin A1c over the course of a year in patients on alirocumab as compared to patients on placebo (all patients were also on a statin). With respect to evolocumab, I found the following data:
1. A 52-week placebo-controlled trial of evolocumab in patients with hyperlipidemia published in the New England Journal of Medicine in 2014 found the mean change from baseline for fasting glucose at week 52 was 1.3 mg per deciliter for evolocumab and 0.4 mg per deciliter for placebo. The mean change from baseline for HbA1c at week 52 was 0.02% for evolocumab and 0.00% for placebo (table 3 and supplementary table S3).
1. The Osler trials recently published in the New England Journal of Medicine showed that 1.1% of the patients who received evolocumab developed diabetes, as compared to 0.7% of the patients in the standard of care group.
What do all these small differences add up to? It’s not possible to say yet, but I assume someone will do a meta-analysis at some point, and there may be some discussion of this issue in the FDA review of these agents, which will be posted prior to advisory committee meetings.
I should note that even if PCSK9 inhibitors do increase blood glucose and the risk of developing diabetes, they would still be very worthwhile for patients who are at significant risk of heart attack and stroke, if they are shown to be effective and have acceptable safety.